A) \[b>d>a>c\]
B) \[b>d>c>a\]
C) \[a>b>c>d\]
D) \[b>c>d>a\]
Correct Answer: D
Solution :
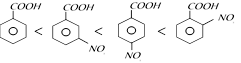 Electron withdrawing group, increases the acidity of benzoic acid, O-isomer will have higher acidity then corresponding m and p-isomer due to ortho effect.
Electron withdrawing group, increases the acidity of benzoic acid, O-isomer will have higher acidity then corresponding m and p-isomer due to ortho effect.
You need to login to perform this action.
You will be redirected in
3 sec
