A) Water has lower freezing point and higher boiling point than benzene
B) It dissociates to a greater extent in benzene than in water
C) It associates in water and dissociates in benzene
D) It dissociates in water and associates in benzene
Correct Answer: D
Solution :
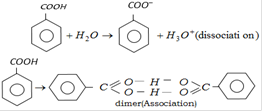 In benzene solution
In benzene solution
You need to login to perform this action.
You will be redirected in
3 sec
