A) \[\frac{6}{RT}\]
B) different from \[{{E}_{a}}\] obtained in laboratory,
C) P is required
D) can't say anything
Correct Answer: B
Solution :
[b] The presence of enzyme (catalyst) increases the speed of reaction by lowering the energy barrier, i.e., a new path is followed with lower activation energy.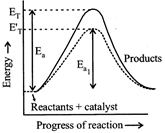 Here \[{{E}_{T}}\] is the threshold energy. \[{{E}_{a}}\] and \[{{E}_{a1}}\] is energy of activation of reaction in absence and presence of catalyst respectively.
Here \[{{E}_{T}}\] is the threshold energy. \[{{E}_{a}}\] and \[{{E}_{a1}}\] is energy of activation of reaction in absence and presence of catalyst respectively.
You need to login to perform this action.
You will be redirected in
3 sec
