| (I) \[{{K}_{3}}\left[ Fe\left( C{{N}_{6}} \right) \right]\] |
| (II) \[{{\left[ Ni{{\left( CO \right)}_{4}} \right]}^{10}}\] |
| (III) \[{{\left[ Cr{{(N{{H}_{3}})}_{6}} \right]}^{3+}}\] |
| (IV) \[{{\left[ Mn\,(C{{N}_{6}}) \right]}^{4\,-}}\] |
| Choose the correct code: |
A) I only
B) II and III
C) I and IV
D) IV only
Correct Answer: C
Solution :
[c] \[F{{e}^{3+}}\,in\,{{\left[ Fe(C{{N}_{6}}) \right]}^{3-}}\]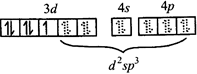 \[\Rightarrow \] low spin complex \[\mu =1.732BM\] Ni in \[\left[ Ni{{\left( CO \right)}_{4}} \right]\]
\[\Rightarrow \] low spin complex \[\mu =1.732BM\] Ni in \[\left[ Ni{{\left( CO \right)}_{4}} \right]\] 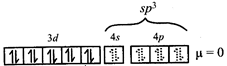 \[C{{r}^{3+}}\,in{{[Cr{{\left( N{{H}_{3}} \right)}_{6}}]}^{3+}}\]
\[C{{r}^{3+}}\,in{{[Cr{{\left( N{{H}_{3}} \right)}_{6}}]}^{3+}}\] 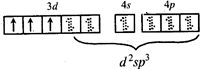 \[\Rightarrow \] low and high spin complex is applicable for \[{{d}^{4}}\] to \[{{d}^{7}}\] configuration \[M{{n}^{2+}}in{{\left[ Mn{{\left( CN \right)}_{6}} \right]}^{4-}}\]
\[\Rightarrow \] low and high spin complex is applicable for \[{{d}^{4}}\] to \[{{d}^{7}}\] configuration \[M{{n}^{2+}}in{{\left[ Mn{{\left( CN \right)}_{6}} \right]}^{4-}}\] 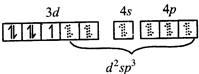 \[\Rightarrow \] low spin complex \[\mu =1.732\,BM\]
\[\Rightarrow \] low spin complex \[\mu =1.732\,BM\]
You need to login to perform this action.
You will be redirected in
3 sec
