A) \[{{\left[ MnC{{l}_{6}} \right]}^{3-}}\] is more paramagnetic than \[{{\left[ Mn{{\left( CN \right)}_{6}} \right]}^{3-}}\]
B) Both \[{{[Co{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{3-}}\]and \[{{[Co{{F}_{6}}]}^{3-}}\]are paramagnetic.
C) \[{{[Fe{{(CN)}_{6}}]}^{3-}}\] forms inner orbital complex whereas \[{{\left[ Fe{{F}_{6}} \right]}^{3-}}\] forms outer orbital complex.
D) Both [a] and [b]
Correct Answer: B
Solution :
[b] \[{{[Co{{({{C}_{2}}{{O}_{4}})}_{3}}]}^{3-}}\] is diamagnetic as oxalate is a strong ligand causing pairing of 3d electrons in \[C{{o}^{3+}}\] thereby leading to \[{{d}^{2}}s{{p}^{3}}\] hybridisation.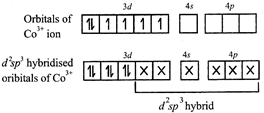
You need to login to perform this action.
You will be redirected in
3 sec
