A) tetrahedral and tetrahedral
B) square planar and square planar
C) tetrahedral and square planar
D) square planar and tetrahedral
Correct Answer: C
Solution :
[c] In both states (paramagnetic and diamagnetic) of the given complex, Ni exists as \[N{{i}^{2+}}\] whose electronic configuration is \[[Ar]3{{d}^{8}}4{{s}^{0}}\].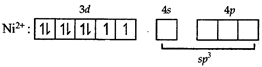 In the above paramagnetic state, geometry of the complex is \[s{{p}^{3}}\] giving tetrahedral geometry. The diamagnetic state is achieved by pairing of electrons in 3d orbital.
In the above paramagnetic state, geometry of the complex is \[s{{p}^{3}}\] giving tetrahedral geometry. The diamagnetic state is achieved by pairing of electrons in 3d orbital. 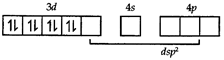 Thus the geometry of the complex will be \[ds{{p}^{2}}\] giving square planar geometry.
Thus the geometry of the complex will be \[ds{{p}^{2}}\] giving square planar geometry.
You need to login to perform this action.
You will be redirected in
3 sec
