A) \[Ni{{\left( CO \right)}_{4}}\]
B) \[{{[NiC{{l}_{4}}]}^{2-}}\]
C) \[Ni{{\left( PP{{h}_{3}} \right)}_{4}}\]
D) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
Correct Answer: B
Solution :
[b] \[{{\left[ NiC{{l}_{4}} \right]}^{2-}},O.S.of\,Ni=+2\] \[Ni(28)=3{{d}^{8}}4{{s}^{2}}\]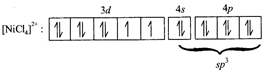 No. of unpaired electrons = 2 Magnetic moment, \[\mu =2.82\text{ }BM.\]
No. of unpaired electrons = 2 Magnetic moment, \[\mu =2.82\text{ }BM.\]
You need to login to perform this action.
You will be redirected in
3 sec
