A) Red complex has a square planar geometry.
B) Complex has symmetrical H-bonding
C) Red complex has a tetrahedral geometry.
D)
Dimethylglyoxime functions as bidentate ligand. 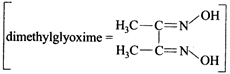
Correct Answer: C
Solution :
[c] Nickel ions are frequently detected by the formation of red precipitate of the complex of nickel dimethylglyoxime, when heated with dimethylglyoxime.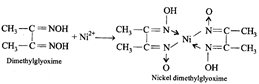
You need to login to perform this action.
You will be redirected in
3 sec
