A) \[{{d}^{2}}s{{p}^{3}}\] hybridised and diamagnetic
B) \[s{{p}^{3}}{{d}^{2}}\] hybridised and paramagnetic
C) \[s{{p}^{3}}{{d}^{2}}\] hybridised and diamagnetic
D) \[{{d}^{2}}s{{p}^{3}}\] hybridised and paramagnetic
Correct Answer: D
Solution :
[d] \[F{{e}^{2+}}:\]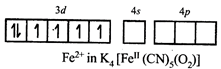
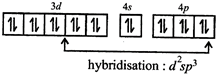 Complex is paramagnetic due topresence of unpaired electron at \[O_{2}^{-}\] i.e., superoxide acting as ligand.
Complex is paramagnetic due topresence of unpaired electron at \[O_{2}^{-}\] i.e., superoxide acting as ligand.
You need to login to perform this action.
You will be redirected in
3 sec
