A) \[197\text{ }c{{m}^{2}}oh{{m}^{-1}}e{{q}^{-1}}\]
B) \[172\,c{{m}^{2}}oh{{m}^{-1}}e{{q}^{-1}}\]
C) \[135.5\,c{{m}^{2}}oh{{m}^{-1}}e{{q}^{-1}}\]
D) \[160.5\,c{{m}^{2}}oh{{m}^{-1}}e{{q}^{-1}}\]
Correct Answer: C
Solution :
[c] Total charge =2 Number of equivalent of ion \[=\frac{Charge\text{ }on\text{ }the\text{ }ion}{Total\text{ }charge}\] \[\therefore Eq\] of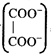 \[=\frac{2}{2}=1\] Eq of \[N{{a}^{+}}=\frac{1}{2},\text{ }Eq\text{ }of\,{{K}^{+}}=\frac{1}{2}\]
\[=\frac{2}{2}=1\] Eq of \[N{{a}^{+}}=\frac{1}{2},\text{ }Eq\text{ }of\,{{K}^{+}}=\frac{1}{2}\] 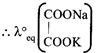
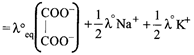 \[=74+\frac{50}{2}+\frac{73}{2}=135.5\text{ }oh{{m}^{-1}}c{{m}^{2}}e{{q}^{-1}}\]
\[=74+\frac{50}{2}+\frac{73}{2}=135.5\text{ }oh{{m}^{-1}}c{{m}^{2}}e{{q}^{-1}}\]
You need to login to perform this action.
You will be redirected in
3 sec
