| The e.m.f. of a Daniell cell at 298 K is \[{{E}_{1}}.\] |
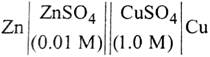 |
| When the concentration of \[ZnS{{O}_{4}}\] is 1.0 M and that of \[CuS{{O}_{4}}\] is 0.01 M, the e.m.f. changed to \[{{E}_{2}}\]. What is the relationship between \[{{E}_{1}}\] and\[{{E}_{2}}\]? |
A) \[{{E}_{2}}=0\ne {{E}_{1}}\]
B) \[{{E}_{1}}>{{E}_{2}}\]
C) \[{{E}_{1}}<{{E}_{2}}\]
D) \[{{E}_{1}}={{E}_{2}}\]
Correct Answer: B
Solution :
[b] Cell reaction is, \[Zn+C{{u}^{2+}}\to Z{{n}^{2+}}+Cu\] \[{{E}_{cell}}=E_{_{Cell}}^{{}^\circ }-\frac{RT}{nF}\ell n\frac{[Z{{n}^{2+}}]}{[C{{u}^{2+}}]}\] Greater the factor \[\left[ \frac{(Z{{n}^{2+}})}{(C{{u}^{2+}})} \right]\], less is the EMF Hence \[{{E}_{1}}>{{E}_{2}}\]You need to login to perform this action.
You will be redirected in
3 sec
