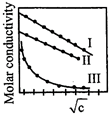
A) \[I(NaCl),II(HCL),III,(N{{H}_{4}}OH)\]
B) \[I\left( HCl \right),II\left( NaCl \right),III,\left( N{{H}_{4}}OH \right)\]
C) \[I\left( N{{H}_{4}}OH \right),II\left( NaCl \right),III,\left( HCl \right)\]
D) \[I(N{{H}_{4}}OH),II(HCl),III,(NaCl)\]
Correct Answer: B
Solution :
[b] Ionic molar conductivity of \[{{H}^{+}}\] is very high and \[N{{H}_{4}}OH\] is a weak electrolyte.You need to login to perform this action.
You will be redirected in
3 sec
