A) 0.10
B) 0.50
C) 0.75
D) 0.54
Correct Answer: C
Solution :
[c] Initial At eqn.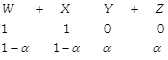 \[{{K}_{ep}}=9=\frac{{{\alpha }^{2}}}{{{(1-\alpha )}^{2}}}\] \[\therefore \text{ }\alpha =0.75\] Moles of \[Y=0.75\]
\[{{K}_{ep}}=9=\frac{{{\alpha }^{2}}}{{{(1-\alpha )}^{2}}}\] \[\therefore \text{ }\alpha =0.75\] Moles of \[Y=0.75\]
You need to login to perform this action.
You will be redirected in
3 sec
