A) 8
B) 4
C) 12
D) 6
Correct Answer: B
Solution :
[b]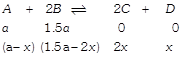 Hence \[{{K}_{C}}=\frac{{{(2x)}^{2}}\times x}{(a-x){{(1.5a-2x)}^{2}}}\] Given, at equilibrium \[\therefore ~\left( a-x \right)=\left( 1.5a-2x \right)\] \[\therefore ~a=2x\] On solving \[{{K}_{C}}=4\]
Hence \[{{K}_{C}}=\frac{{{(2x)}^{2}}\times x}{(a-x){{(1.5a-2x)}^{2}}}\] Given, at equilibrium \[\therefore ~\left( a-x \right)=\left( 1.5a-2x \right)\] \[\therefore ~a=2x\] On solving \[{{K}_{C}}=4\]
You need to login to perform this action.
You will be redirected in
3 sec
