A) 0.36
B) 0.675
C) 0.45
D) 0.54
Correct Answer: B
Solution :
[b] Initial At equilibrium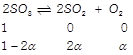 \[\therefore 2\alpha =0.6,\text{ }\therefore \alpha =0.3\] \[\left[ S{{O}_{3}} \right]=1-2\alpha =1-0.6=0.4\] \[\left[ S{{O}_{2}} \right]=2\alpha =0.6\] \[\left[ {{O}_{2}} \right]=\alpha =0.3\] \[{{K}_{eq}}=\frac{\alpha \times {{(2\alpha )}^{2}}}{{{(1-2\alpha )}^{2}}}=\frac{0.3\times 0.6\times 0.6}{0.4\times 0.4}=0.675\]
\[\therefore 2\alpha =0.6,\text{ }\therefore \alpha =0.3\] \[\left[ S{{O}_{3}} \right]=1-2\alpha =1-0.6=0.4\] \[\left[ S{{O}_{2}} \right]=2\alpha =0.6\] \[\left[ {{O}_{2}} \right]=\alpha =0.3\] \[{{K}_{eq}}=\frac{\alpha \times {{(2\alpha )}^{2}}}{{{(1-2\alpha )}^{2}}}=\frac{0.3\times 0.6\times 0.6}{0.4\times 0.4}=0.675\]
You need to login to perform this action.
You will be redirected in
3 sec
