A) 4.56
B) 4.73
C) 9.45
D) 6.78
Correct Answer: A
Solution :
[a]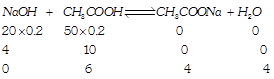 \[Conc.=\frac{\text{Millimoles}}{\text{Total}\,\text{Volume}}\] \[[C{{H}_{3}}COOH]=\frac{6}{70};[C{{H}_{3}}COONa]=\frac{4}{70}\] \[pH=-log1.8\times {{10}^{-5}}+log\frac{4/70}{6/70},pH=4.56\]
\[Conc.=\frac{\text{Millimoles}}{\text{Total}\,\text{Volume}}\] \[[C{{H}_{3}}COOH]=\frac{6}{70};[C{{H}_{3}}COONa]=\frac{4}{70}\] \[pH=-log1.8\times {{10}^{-5}}+log\frac{4/70}{6/70},pH=4.56\]
You need to login to perform this action.
You will be redirected in
3 sec
