A) \[{{K}_{P}}=\frac{{{\alpha }^{2}}}{1+{{\alpha }^{2}}P}\]
B) \[{{K}_{P}}=\frac{{{\alpha }^{2}}{{P}^{2}}}{1-{{\alpha }^{2}}}\]
C) \[{{K}_{P}}=\frac{{{P}^{2}}}{1-{{\alpha }^{2}}}\]
D) \[{{K}_{P}}=\frac{{{\alpha }^{2}}P}{1-{{\alpha }^{2}}}\]
Correct Answer: D
Solution :
[d]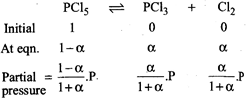 Total mole\[=1-\alpha +\alpha +\alpha =1+\alpha \] \[{{K}_{p}}=\frac{\frac{\alpha }{1+\alpha }P.\frac{\alpha }{1+\alpha }.P}{\frac{1-\alpha }{1+\alpha }P}=\frac{{{\alpha }^{2}}P}{1-{{\alpha }^{2}}}\]
Total mole\[=1-\alpha +\alpha +\alpha =1+\alpha \] \[{{K}_{p}}=\frac{\frac{\alpha }{1+\alpha }P.\frac{\alpha }{1+\alpha }.P}{\frac{1-\alpha }{1+\alpha }P}=\frac{{{\alpha }^{2}}P}{1-{{\alpha }^{2}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
