A) Straight line parallel to x-axis.
B) Straight line with a negative slope.
C) Curve starting at origin.
D) Straight line passing through origin.
Correct Answer: B
Solution :
[b] According to Boyle's law, PV = constant \[\therefore ~log\,P+log\,V=constant\] \[log\,P=-\text{ }log\,V+constant\] Hence, the plot of log P vs log V is straight line with negative slope.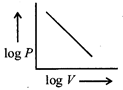
You need to login to perform this action.
You will be redirected in
3 sec
