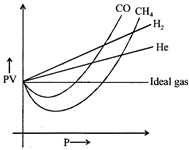
A) Intermolecular interactions tor \[{{H}_{2}}\] and He are very low.
B) Molecular size or atomic size for \[{{H}_{2}}\] and He is small.
C) Both [a] and [b]
D) Neither [a] nor [b]
Correct Answer: C
Solution :
[c] Due to small size of these species (\[{{H}_{2}}\]and He) intermolecular interactions (van der Waal forces) are very low, therefore it is difficult to compress these.You need to login to perform this action.
You will be redirected in
3 sec
