A) 8.96 L
B) 4.48 L
C) 6.72 L
D) 2.24 L
Correct Answer: A
Solution :
[a]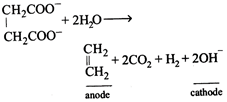 Total equivalent of \[{{C}_{2}}{{H}_{4}}+C{{O}_{2}}+{{H}_{2}}=0.2+0.2+0.2=0.6\] Total moles of gases \[=\frac{0.2}{2}+\frac{0.2}{1}+\frac{0.2}{2}=0.4\] \[\text{V=}\frac{nRT}{P}=\frac{0.4\times 0.0821\times 273}{1}=8.96L.\]
Total equivalent of \[{{C}_{2}}{{H}_{4}}+C{{O}_{2}}+{{H}_{2}}=0.2+0.2+0.2=0.6\] Total moles of gases \[=\frac{0.2}{2}+\frac{0.2}{1}+\frac{0.2}{2}=0.4\] \[\text{V=}\frac{nRT}{P}=\frac{0.4\times 0.0821\times 273}{1}=8.96L.\]
You need to login to perform this action.
You will be redirected in
3 sec
