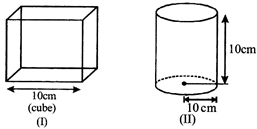
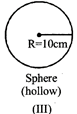
 If all the containers are placed at the same temperatures, then find the incorrect options -
If all the containers are placed at the same temperatures, then find the incorrect options -
A) Pressure of the gas is minimum in (III) container
B) Pressure of the gas is equal in I and II container
C) Pressure of the gas is maximum in (I)
D) The ratio of pressure in II and III container is 4:3
Correct Answer: B
Solution :
[b] n, T same hence \[P\propto \frac{1}{V},\] \[{{V}_{1}}=1000\,c{{m}^{3}}\] \[{{V}_{2}}=\pi {{(10)}^{2}}\times 10=1000\pi c{{m}^{3}}\] \[{{V}_{3}}=\frac{4}{3}\pi {{(10)}^{3}}=\frac{4}{3}\pi \,1000\,c{{m}^{3}}\] \[\therefore \] Pressure of the gas is minimum in (III) container. Pressure of the gas is maximum in (I),You need to login to perform this action.
You will be redirected in
3 sec
