A) \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\] is less soluble than \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\].
B) \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\] is primary standard in volumetry.
C) \[CrO{{_{4}^{2-}}^{~}}\] is tetrahedral in shape.
D) \[C{{r}_{2}}{{O}_{7}}^{2-}\] has a \[Cr-O-Cr\] bond.
Correct Answer: A
Solution :
[a]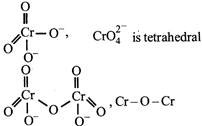 bond in \[C{{r}_{2}}O_{7}^{2-}\] \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\] is used an primary standard in volumetry. Hydration energy of \[N{{a}^{+}}\] is greater than \[{{K}^{\text{+}}}\] because of smaller size of \[N{{a}^{+}},N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\] is more soluble than \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}.\]
bond in \[C{{r}_{2}}O_{7}^{2-}\] \[N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\] is used an primary standard in volumetry. Hydration energy of \[N{{a}^{+}}\] is greater than \[{{K}^{\text{+}}}\] because of smaller size of \[N{{a}^{+}},N{{a}_{2}}C{{r}_{2}}{{O}_{7}}\] is more soluble than \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}.\]
You need to login to perform this action.
You will be redirected in
3 sec
