A) \[CO_{3}^{2-}~<C{{O}_{2}}<CO\]
B) \[C{{O}_{2}}<CO_{3}^{2-}<CO\]
C) \[CO<CO_{3}^{2-}<C{{O}_{2}}\]
D) \[CO<C{{O}_{2}}<CO_{3}^{2-}\]
Correct Answer: D
Solution :
[d] Structures of CO, \[C{{O}_{2}}\] and \[CO_{3}^{2-}\] are: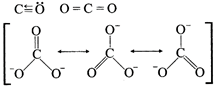
| Compound | CO | \[C{{O}_{2}}\] | \[C{{O}_{3}}\] |
| Bond order | 3 | 2 | 1.33 |
You need to login to perform this action.
You will be redirected in
3 sec
