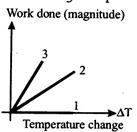
A) Isobaric, adiabatic isochoric
B) Adiabatic, isobaric, isochoric
C) Isochoric, adiabatic, isobaric
D) Isochoric, isobaric, adiabatic
Correct Answer: D
Solution :
[d] Isochoric proceess \[dV=0\] \[W=0\]proceess 1 Isobaric: \[W=P\Delta V=nRAT\] Adiabatic \[|W|=\frac{nR\Delta T}{\gamma -1}0<\gamma -1<1\] As work done in case of adiabatic process is more so process 3 is adiabatic and process 2 is isobaric.You need to login to perform this action.
You will be redirected in
3 sec
