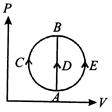
A) greater in process (ii) than in (i)
B) the least in process (ii)
C) the same in (i) and (iii)
D) less in (iii) than in (ii)
Correct Answer: D
Solution :
[d] Heat absorbed by gas in three processes is given by \[{{Q}_{ACB}}=\Delta U+{{W}_{ACB}}\] \[{{Q}_{ACB}}=\Delta U\] \[{{Q}_{ACB}}=\Delta U+{{W}_{AEB}}\] The change in internal energy in all the three cases is same and \[{{W}_{ACB}}\] is \[+ve,\] \[{{W}_{AEB}}\] is\[-ve\]. Hence \[{{Q}_{ACB}}>{{Q}_{ADB}}>{{Q}_{AEB}}\]You need to login to perform this action.
You will be redirected in
3 sec
