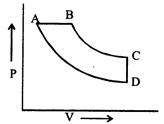
A) BA, AD, DC, CB
B) DC, CB, BA, AD
C) AB, BC, CD, DA
D) CD, DA, AB, BC
Correct Answer: D
Solution :
[d] From C to D, V is constant. So process is isochoric. From D to A, the curve represents constant temperature. So the process is isothermal. From A to B, pressure is constant. So, the process is isobaric. BC represents constant entropy.You need to login to perform this action.
You will be redirected in
3 sec
