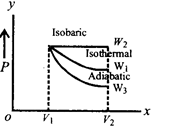
A) \[{{W}_{2}}>{{W}_{1}}>{{W}_{3}}\]
B) \[{{W}_{2}}>{{W}_{3}}>{{W}_{1}}\]
C) \[{{W}_{1}}>{{W}_{2}}>{{W}_{3}}\]
D) \[{{W}_{1}}>{{W}_{3}}>{{W}_{2}}\]
Correct Answer: A
Solution :
[a] Work done is equal to area under the curve on PV diagram.You need to login to perform this action.
You will be redirected in
3 sec
