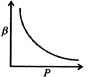
A)
B)
C)
D)
Correct Answer: A
Solution :
[a] PV= constant. Differentiating, \[\frac{PdV}{dP}=-V;\beta =-\left( \frac{1}{V} \right)\left( \frac{dV}{dP} \right)=\left( \frac{1}{P} \right)\Rightarrow \beta \times P=1\] \[\therefore \]Graph between P and P will be a rectangular hyperbola.You need to login to perform this action.
You will be redirected in
3 sec
