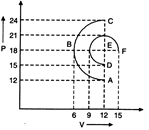
A) ABC
B) DEF
C) Equal in both processes
D) It cannot be predicted
Correct Answer: B
Solution :
[b] \[{{W}_{ABC}}=\frac{\pi {{r}^{2}}}{2}=\frac{\pi {{(6)}^{2}}}{2}=18\pi \] \[{{W}_{DEF}}=\frac{\pi \times {{3}^{2}}}{2}+\frac{\pi \times {{3}^{2}}}{4}+\left( 15-12 \right)\times 18\] \[=6.75\pi +54\] \[{{W}_{DEF}}>{{W}_{ABC}}\]You need to login to perform this action.
You will be redirected in
3 sec
