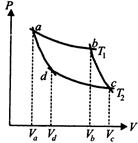
A) \[\frac{{{V}_{b}}}{{{V}_{c}}}\]
B) \[\frac{{{V}_{c}}}{{{V}_{b}}}\]
C) \[\frac{{{V}_{d}}}{{{V}_{a}}}\]
D) \[{{V}_{b}}{{V}_{c}}\]
Correct Answer: A
Solution :
[a] We know that \[\frac{{{V}_{a}}}{{{V}_{b}}}=\frac{{{V}_{d}}}{{{V}_{c}}}\Rightarrow \frac{{{V}_{a}}}{{{V}_{d}}}=\frac{{{V}_{b}}}{{{V}_{c}}}\]You need to login to perform this action.
You will be redirected in
3 sec
