A) \[{{Q}_{1}}>{{Q}_{2}}>{{Q}_{3}}>{{Q}_{4}}\]
B) \[{{Q}_{1}}>{{Q}_{2}}>{{Q}_{4}}>{{Q}_{3}}\]
C) \[{{Q}_{1}}>{{Q}_{4}}>{{Q}_{2}}>{{Q}_{3}}\]
D) \[{{Q}_{1}}<{{Q}_{2}}<{{Q}_{3}}<{{Q}_{4}}\]
Correct Answer: B
Solution :
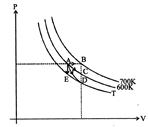
[b] In process-1 heat supplied = area under AB curve\[~+n\times {{C}_{V}}\times 100\] (isobaric process) In process-2 heat supplied = area under AC curve (isothermal process) In process-3 heat supplied = 0 (adiabatic process) In process-4 heat supplied \[=n\times {{C}_{v}}(T-600)\](isobaric process)You need to login to perform this action.
You will be redirected in
3 sec
