A) \[\frac{a}{2}\]
B) \[\frac{a}{2\sqrt{2}}\]
C) \[\frac{\sqrt{3}}{4}a\]
D) \[\frac{\sqrt{3}}{2}a\]
Correct Answer: B
Solution :
For the fcc structure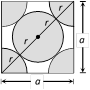 \[4r={{({{a}^{2}}+{{a}^{2}})}^{1/2}}\]\[=a\sqrt{2}\] Þ \[r=\frac{a\sqrt{2}}{4}=\frac{a}{2\sqrt{2}}\]
\[4r={{({{a}^{2}}+{{a}^{2}})}^{1/2}}\]\[=a\sqrt{2}\] Þ \[r=\frac{a\sqrt{2}}{4}=\frac{a}{2\sqrt{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
