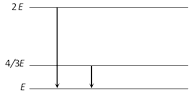
 |
A) \[\lambda /3\]
B) \[3\lambda /4\]
C) \[4\lambda /3\]
D) \[3\lambda \]
Correct Answer: D
Solution :
| [d] \[2E-E=\frac{hc}{\lambda }\Rightarrow E=\frac{hc}{\lambda }\] |
| \[\frac{4E}{3}-E=\frac{hc}{\lambda '}\Rightarrow \frac{E}{3}=\frac{hc}{\lambda '}\ \ \therefore \frac{\lambda '}{\lambda }=3\Rightarrow \lambda '=3\lambda \] |
You need to login to perform this action.
You will be redirected in
3 sec
