| Which of the following option is correct about \[N{{O}_{2}}\And Cl{{O}_{2}}?\] |
| (i) Both are paramagnetic species |
| (ii) Both compounds dimerised readily |
| (iii) Both have \[s{{p}^{2}}\] hybridisation |
| (iv) Both have Bent shape. |
A) (i), (ii)
B) (i), (iii), (iv)
C) (ii), (iii), (iv)
D) only (i)
Correct Answer: B
Solution :
[b]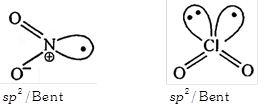 \[Cl{{O}_{2}}\] doesn't dimerised readily between odd electron delocalized in a vacant 3d orbital.
\[Cl{{O}_{2}}\] doesn't dimerised readily between odd electron delocalized in a vacant 3d orbital.
You need to login to perform this action.
You will be redirected in
3 sec
