A) \[{{V}_{1}}={{V}_{2}}>{{V}_{3}}={{V}_{4}}\]
B) \[{{V}_{1}}={{V}_{2}}<{{V}_{3}}={{V}_{4}}\]
C) \[{{V}_{1}}={{V}_{2}}={{V}_{3}}={{V}_{4}}\]
D) \[{{V}_{4}}>{{V}_{3}}>{{V}_{2}}>{{V}_{1}}\]
Correct Answer: A
Solution :
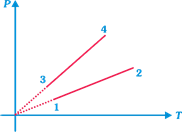
| [a] In isochoric process, \[V=\] constant or \[P\alpha T\] i.e., P-T graph is a straight line passing through origin. But since \[P=(nRT)\frac{1}{V}\] |
| Slope of the straight line \[\alpha \frac{1}{V}\] |
| \[{{(\text{slope)}}_{12}}<{{(\text{slope)}}_{34}}\] |
| \[{{V}_{1}}={{V}_{2}}>{{V}_{3}}={{V}_{4}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
