A) 40 J
B) 80 J
C) 120 J
D) 60 J
Correct Answer: C
Solution :
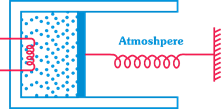
| [c]Work done \[=\int_{0}^{{{x}_{0}}}{{{p}_{0}}Axdx}+\frac{kx_{0}^{2}}{2}\] |
| \[={{p}_{0}}A{{x}_{0}}+\frac{kx_{0}^{2}}{2}=80+40=120J\] |
| where p = pressure of gas, \[{{p}_{0}}=\] atmospheric pressure k = spring constant \[{{x}_{0}}=\] compression in spring. |
You need to login to perform this action.
You will be redirected in
3 sec
