Answer:
Figure
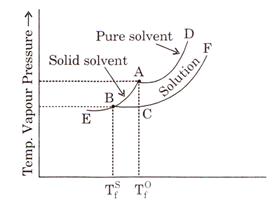
explains diagrammatically the depression of freezing point of a liquid.
![]() is
the freezing point of the pure liquid. EBA is the sublimation curve for the
solid solvent. 1
AD is the vapour pressure curve for pure liquid. The point
at which these two curves meet is the freezing point of the pure liquid.
is
the freezing point of the pure liquid. EBA is the sublimation curve for the
solid solvent. 1
AD is the vapour pressure curve for pure liquid. The point
at which these two curves meet is the freezing point of the pure liquid.
 2
Fig.
: Depression of freezing point.
Numerical: Molality of solution:
2
Fig.
: Depression of freezing point.
Numerical: Molality of solution:
![]() 1
1
![]() 1
1
You need to login to perform this action.
You will be redirected in
3 sec
