Answer:
(i)
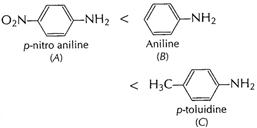 ?
?![]() is
electron-withdrawing group, decreases electron-density at N in ?
is
electron-withdrawing group, decreases electron-density at N in ?![]() hence
(A) less basic than aniline. ?
hence
(A) less basic than aniline. ?![]() is
electron-donating group increases electron density at N in ?
is
electron-donating group increases electron density at N in ?![]() hence (C)
more basic than aniline.
(ii)
hence (C)
more basic than aniline.
(ii) ![]() As alkyl/aryl
part (hydrophobic part) increases solubility decreases. Solubility of 2° amine
is less than that of 1 ° amine.
As alkyl/aryl
part (hydrophobic part) increases solubility decreases. Solubility of 2° amine
is less than that of 1 ° amine.
You need to login to perform this action.
You will be redirected in
3 sec
