Answer:
(a)
The electrode potential of ![]() is
maximum.
is
maximum. ![]() . That is
why it is the strongest oxidizing
agent. 1
(b)
According to Bronsted Lowry concept, a strong acid has a weak conjugate base
and a weak acid has a strong conjugate base. Thus the conjugate base of the
oxoacid is:
. That is
why it is the strongest oxidizing
agent. 1
(b)
According to Bronsted Lowry concept, a strong acid has a weak conjugate base
and a weak acid has a strong conjugate base. Thus the conjugate base of the
oxoacid is:
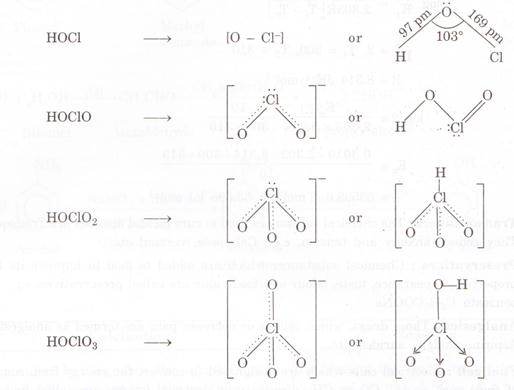 The
basicity of these conjugate bases are in the following manner:
The
basicity of these conjugate bases are in the following manner:
![]() Thus,
the order of acidity of the acids will be
Thus,
the order of acidity of the acids will be
![]() 1
1
You need to login to perform this action.
You will be redirected in
3 sec
