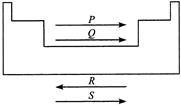
A)
P Q R S Lonisation enthalpy Electron gain enthalpy Atomic radius Electro negativity
B)
P Q R S Atomic radius Lonisation enthalpy Electro negativity Electron gain enthalpy
C)
P Q R S Ionisation enthalpy Atomic radius Electro negativity Electron gain enthalpy
D)
P Q R S Electro negativity Electron gain enthalpy Ionisation enthalpy Atomic radius
Correct Answer: A
Solution :
Ionisation enthalpy, electron gain enthalpy and electronegativity increases in a period from left to right while atomic radius decreases.You need to login to perform this action.
You will be redirected in
3 sec
