A) The electrophile in both cases is \[C{{H}_{2}}=O\].
B) The electrophile in both cases is \[C{{H}_{2}}=\overset{+}{\mathop{O}}\,H\].
C) The electrophile is \[C{{H}_{2}}=O\]in the presence of an alkali and \[C{{H}_{2}}=\overset{+}{\mathop{O}}\,H\]in the presence of an acid.
D) It is a nucleophilic substitution reaction.
Correct Answer: C
Solution :
[c]: Condensation of phenol with formaldehyde is an electrophilic substitution reaction. Base converts phenol into p hen oxide ion which being more reactive, reacts easily with \[C{{H}_{2}}=O\](a weak electrophile).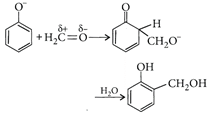 In presence of an acid, \[C{{H}_{2}}=O\](a weak + electrophile) is protonated to \[C{{H}_{2}}=\overset{+}{\mathop{O}}\,H\](a strong electrophile) which easily reacts with phenol (a weak nucleophile).
In presence of an acid, \[C{{H}_{2}}=O\](a weak + electrophile) is protonated to \[C{{H}_{2}}=\overset{+}{\mathop{O}}\,H\](a strong electrophile) which easily reacts with phenol (a weak nucleophile). 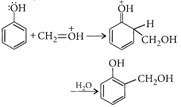
You need to login to perform this action.
You will be redirected in
3 sec
