A)
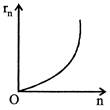
B)
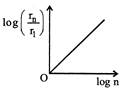
C)
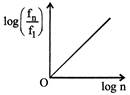
D) Both (a) and (b)
Correct Answer: D
Solution :
Radius of \[{{n}^{th}}\] orbit \[{{r}_{n}}\propto {{n}^{2}},\] graph between \[{{r}_{n}}\] and n is a parabola. Also, \[\frac{{{r}_{n}}}{{{r}_{1}}}={{\left( \frac{n}{1} \right)}^{2}}\Rightarrow {{\log }_{e}}\left( \frac{{{r}_{n}}}{{{r}_{1}}} \right)=2{{\log }_{e}}(n)\] Comparing this equation with \[y=mx+c,\] Graph between \[{{\log }_{e}}\left( \frac{{{r}_{n}}}{{{r}_{1}}} \right)\] and \[{{\log }_{e}}(n)\] will be a straight line, passing from origin. Similarly it can be proved that graph between \[{{\log }_{e}}\left( \frac{{{f}_{n}}}{{{f}_{1}}} \right)\] and \[{{\log }_{e}}n\]is a straight line. But with negative slops.You need to login to perform this action.
You will be redirected in
3 sec
