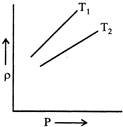
A) \[{{T}_{1}}>{{T}_{2}}\]
B) \[{{T}_{2}}>{{T}_{1}}\]
C) \[{{T}_{1}}={{T}_{2}}\]
D) All the three possible
Correct Answer: B
Solution :
According to ideal gas equation \[PV=nRT\] \[PV=\frac{m}{M}RT,\] \[P=\frac{\rho }{M}RT,\] or \[\frac{\rho }{P}=\frac{M}{RT}\] or \[\frac{\rho }{P}\propto \frac{1}{T}\] Here, \[\frac{\rho }{P}\] represent the slope of graph. Hence, \[{{T}_{2}}>{{T}_{1}}\]You need to login to perform this action.
You will be redirected in
3 sec
