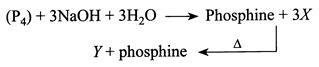 The reaction type, the oxidation state of P in species X and Y are respectively.
The reaction type, the oxidation state of P in species X and Y are respectively.
A) Redox reaction, \[-1\] and \[-5\]
B) Disproportionation reaction, \[+1\] and \[+5\]
C) Comproportionation reaction, \[+1\] and\[+3\]
D) Disproportionation reaction,\[-1\] and \[+5\]
Correct Answer: B
Solution :
[b] \[X=\overset{+1}{\mathop{Na}}\,\overset{+2}{\mathop{{{H}_{2}}}}\,\overset{+1}{\mathop{P}}\,{{O}_{2}}^{-4}\] O.S. of \[P=+1\] Sodium hypophosphite. \[Y=\overset{+3}{\mathop{Na}}\,\overset{+5}{\mathop{P}}\,{{O}_{4}}^{-8}\] O.S. of \[P=+5\] Sodium phosphate Reaction is disproportionationYou need to login to perform this action.
You will be redirected in
3 sec
