A) \[30%\]
B) \[60%\]
C) \[0.2%\]
D) \[20%\]
Correct Answer: D
Solution :
[d] First method Pressure at \[200\text{ }K=2\text{ }atm\] Pressure at \[250\text{ }K=p\text{ }atm\] Using relation; \[\frac{{{P}_{1}}}{{{T}_{1}}}=\frac{{{P}_{2}}}{{{T}_{2}}}\Rightarrow \frac{P}{250}=\frac{2}{200}\] \[\therefore \,\,\,\,P=2.5\,atm\]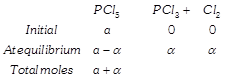
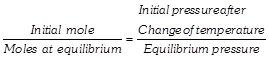 \[\frac{a}{a+\alpha }=\frac{2.5}{3}\Rightarrow \,\,\,\alpha =\frac{1}{5}a\] Therefore, % of \[PC{{l}_{5}}\] dissociated \[=\frac{\alpha }{a}\times 100\] \[=\frac{a}{5\times a}\times 100=20%\] Using relation: \[\alpha =\frac{{{T}_{1}}{{P}_{2}}-{{T}_{2}}{{P}_{1}}}{{{T}_{2}}{{P}_{1}}}\] \[=\frac{200\times 3-250\times 2}{250\times 2}=\frac{600-500}{500}\] \[\alpha =\frac{1}{5}\] % of \[\alpha =\frac{1}{5}\times 100=20%\]
\[\frac{a}{a+\alpha }=\frac{2.5}{3}\Rightarrow \,\,\,\alpha =\frac{1}{5}a\] Therefore, % of \[PC{{l}_{5}}\] dissociated \[=\frac{\alpha }{a}\times 100\] \[=\frac{a}{5\times a}\times 100=20%\] Using relation: \[\alpha =\frac{{{T}_{1}}{{P}_{2}}-{{T}_{2}}{{P}_{1}}}{{{T}_{2}}{{P}_{1}}}\] \[=\frac{200\times 3-250\times 2}{250\times 2}=\frac{600-500}{500}\] \[\alpha =\frac{1}{5}\] % of \[\alpha =\frac{1}{5}\times 100=20%\]
You need to login to perform this action.
You will be redirected in
3 sec
