A) 1, 2-dichloropropane
B) 1, 1-dichloropropane
C) 1, 3-dichloropropane
D) 2, 2-dichloropropane.
Correct Answer: B
Solution :
[b] :.As the obtained compound reduces Tollens' reagent, it must be an aldehyde. Thus, it is obvious that both the -Cl atoms are present at C - 1. Hence, the compound 'X? is 1, 1-dichloropropane and the reactions are as follows: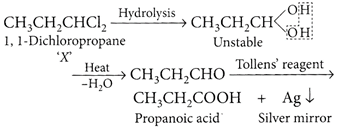
You need to login to perform this action.
You will be redirected in
3 sec
