A) Molarity\[=\frac{1}{2}M\]
B) \[{{x}_{NaOH}}=\frac{9}{1009}\]
C) \[%w/w=10%\]
D) \[%\,w/v=2%\]
Correct Answer: C
Solution :
[c] : Semi-molal \[\Rightarrow \frac{1}{2}m\]or mol \[\text{k}{{\text{g}}^{-1}}\] \[d=1.02g/mL\] 1 kg of water contains\[\frac{1}{2}\]mole of NaOH i.e., 20 g Mass of solution \[=1000+20=1020g\] Volume of solution \[=\frac{1020}{1.02}=1000\,\text{mL}=1\,\text{L}\]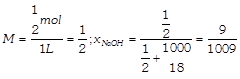 \[%w/w=\frac{20}{1020}\times 100=2%\] \[%w/v=\frac{20}{1000}\times 100=2%\]
\[%w/w=\frac{20}{1020}\times 100=2%\] \[%w/v=\frac{20}{1000}\times 100=2%\]
You need to login to perform this action.
You will be redirected in
3 sec
