A) \[{{({{C}_{2}}{{H}_{5}})}_{3}}CONa+C{{H}_{3}}Cl\]
B) \[C{{H}_{3}}ONa+{{(C{{H}_{3}})}_{3}}CCl\]
C) \[{{(C{{H}_{3}})}_{3}}CONa+{{C}_{2}}{{H}_{5}}Cl\]
D) \[{{(C{{H}_{3}})}_{3}}CONa+C{{H}_{3}}Cl\]
Correct Answer: D
Solution :
[d]: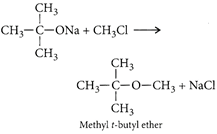
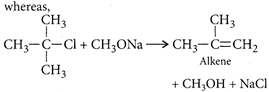 Secondary and tertiary alkyl halides readily undergo elimination reaction rather than ether formation in the presence of alkoxide.
Secondary and tertiary alkyl halides readily undergo elimination reaction rather than ether formation in the presence of alkoxide.
You need to login to perform this action.
You will be redirected in
3 sec
