A) \[BO_{3}^{3-},\,\,CO_{3}^{2-},\,\,NO_{3}^{-}\]
B) \[\operatorname{S}O{{_{3}^{2-}}^{~}},\,CO{{_{3}^{2-}}^{~}},\,\,NO_{3}^{-}\]
C) \[C{{N}^{-}},\,\,{{N}_{2}},\,\,C_{2}^{2-}\]
D) \[\operatorname{PO}{{_{4}^{3-}}^{~}},SO{{_{4}^{2-}}^{~}},ClO_{4}^{-}\]
Correct Answer: B
Solution :
Calculating number of electrons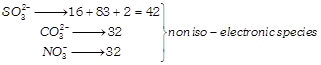
You need to login to perform this action.
You will be redirected in
3 sec
