A) \[{{S}_{2}}O_{4}^{2-}\]
B) \[{{S}_{2}}O_{5}^{2-}\]
C) \[{{S}_{2}}O_{3}^{2-}\]
D) \[{{S}_{2}}O_{7}^{2-}\]
Correct Answer: D
Solution :
In \[{{\operatorname{S}}_{2}}O_{7}^{2-}\] there is an S-O-S bond. Whereas S-S bond is absent as can be seen from the structures given below.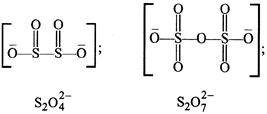
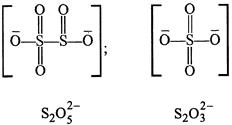
You need to login to perform this action.
You will be redirected in
3 sec
